Three Patents Issued to Magnetecs in January 2011
Inglewood, CA – Magnetecs Corporation, a designer and manufacturer of robotic catheterization control systems for minimally invasive surgical procedures, today reported that the United States Patent Office has issued three patents to the Company related to its robotic Catheter Guidance Control and Imaging (CGCI) system for minimally invasive medical procedures, including catheter ablation for arrhythmia treatment. Magnetecs’ CGCI system is the first to dynamically focus and concentrate electromagnetic energy into a compact, powerful and easy-to-control sphere, akin to how lasers focus light into a precisely directed beam.
The three patents issued to Magnetecs cover:
- The System and Method for Radar-Assisted Catheter Guidance and Control (USPTO Patent No. 7,873,402 B2);
- System and Method for a Magnetic Catheter Tip (USPTO Patent No. 7,873,401 B2 ); and
- Apparatus for Magnetically Deployable Catheter with Mosfet Sensor and Method for Mapping and Ablation (USPTO Patent No. 7,869,854 B2).
In addition, Magnetecs filed eight patent applications in the fourth quarter of 2010. These patent filings supplement the Company’s extensive portfolio of intellectual property. The Company has 52 currently active patents and applications, including 17 patents issued in the U.S., U.K., Italy, France, Germany, Hong Kong, Japan, and China.
“Magnetecs has allocated significant resources to the growth and protection of the Company’s intellectual property portfolio,” said Josh Shachar, Chief Executive Officer and Chief Technology Officer of Magnetecs Corporation. “We have built a powerful and highly defensible patent portfolio in the field of magnetic and robotic guidance and control, and we intend to continue to enhance the substantial value of the Company’s intellectual property.”
CGCI HUMAN STUDIES ONGOING IN MADRID
Human studies using CGCI for patients with arrhythmia, or irregular heartbeat, began on October 7, 2010, at Hospital General Universitario La Paz in Madrid, Spain. The studies are being conducted by Dr. Jose Merino, Director of the Arrhythmia and Electrophysiology Research Unit of the hospital.
To date, 30 patients have participated in the study in which a highly detailed map of the heart is created using the CGCI system’s magnetically guided catheter. The primary outcome of the study, which is expected to be completed during the next few weeks, measures intracardiac anatomic site target acquisition and repetition of acquisition. A description of the study can be found on the ClincalTrials.gov site.
A subsequent study of 40 patients is expected to begin in the second quarter of 2011, in which both mapping and ablation procedures will be conducted using the CGCI system.

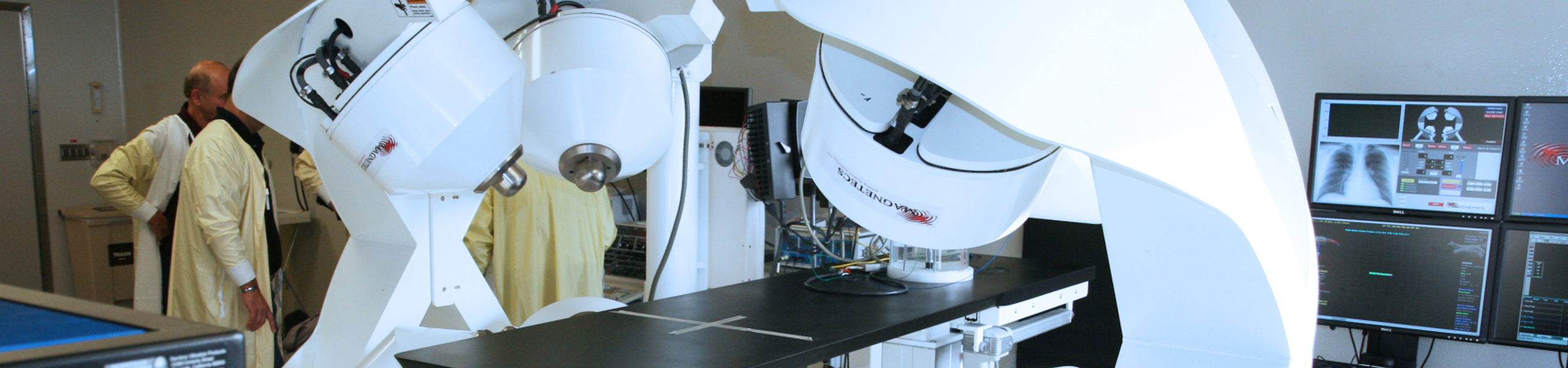
CGCI Installation, La Paz Hospital, Madrid
CGCI SUBMISSION FOR CE MARK CERTIFICATION
CGCI uses an array of eight electromagnets in a unique configuration to intelligently guide a magnetically-tipped catheter. This configuration enables a physician to precisely and consistently control surgical tools in highly dynamic or previously inaccessible environments, while enhancing both the physician’s dexterity and the patient’s safety. The first study focuses on mapping of the heart, which is a diagnostic procedure that is performed for patients who have arrhythmia. Magnetecs expects this study to lead to a CE Mark application for commercialization in Europe in 2011. Additional human studies for ablation are expected to lead to approval of the CGCI system for therapeutic procedures used to correct heart arrhythmia.